Leading the FightAgainst Solid Tumors
About Us
Pioneering Novel Approaches
to Deliver on the Promise of TILs
At Turnstone, our mission is to develop new medicines to treat and cure patients with solid tumors.
Solid tumors present a major burden to society, with high mortality and poor outcomes associated with more advanced disease. Approved immunotherapies represent a significant advancement in the treatment of solid tumors, but many patients either do not respond or experience relapsed disease following an initial response. We believe the most significant challenge to creating curative immunotherapies in these patients is the low numbers of T cells that can recognize and attack the tumor, which we refer to as tumor‑reactive T cells.
To address this problem, we are pioneering a differentiated approach to tumor infiltrating lymphocytes, or TILs. We are developing next-generation TIL therapies by selecting the most potent (meaning able to mediate an anti-tumor response) and tumor-reactive T cells, which we refer to as Selected TILs. We are developing next-generation TIL therapies for potential treatment across multiple solid tumors.
-

One Turnstone
-

One Focus
-

One Fight
Leadership
Experienced Team of
Leaders and Innovators
Turnstone has assembled a seasoned management team, an accomplished Board of Directors and a distinguished Scientific Advisory Board whose contributions to science have meaningfully advanced the field and helped inform our understanding of the relationship between cancer and the immune system.
Our passionate team of leaders comprises world-renowned professionals with deep expertise in TILs, cell therapy, tumor immunology, innate and adaptive immunity, oncolytic viruses, and in the discovery and development, manufacturing, and business and commercial development of complex biologics.

Sammy Farah, PhD, MBA
President & Chief Executive Officer

Stewart Abbot, PhD
Chief Scientific Officer

Saryah Azmat
Chief Business Officer

Mike Burgess, MBChB, PhD
Interim Chief Medical Officer

Vijay Chiruvolu, PhD
Interim Chief Technology Officer

Venkat Ramanan, PhD
Chief Financial Officer

David Stojdl, PhD
SVP, Discovery Research

Chad Green, PhD
SVP, Technical Operations

TJ Langer
SVP, Cell Therapy Development and External Innovation

Adina Pelusio
SVP, Clinical Operations

Karen Major
VP, Regulatory

George Smith, MBA, PhD
VP, Cell Therapy Business Operations

Jerel Davis, PhD
Chairman & Director
Versant Ventures

Mike Burgess, MBChB, PhD
Executive Director
Turnstone Biologics

Sammy Farah, PhD, MBA
Director
Turnstone Biologics

Robert Gould, PhD
Director
Fulcrum Therapeutics

Rishi Gupta, JD
Director
OrbiMed

Kanya Rajangam, MD, PhD
Director
Senti Biosciences

William Waddill
Director
Former CFO Calithera Biosciences, OncoMed, and Ilypsa

Jeff Courtney
In Memoriam
Our Approach
Scientific Resources
Explore academic selection strategies that have demonstrated clinical proof of concept, relevant to Turnstone’s differentiated approach to TIL therapy:
Learn more about our scientific and clinical research, and the potential of Turnstone’s Selected TIL therapy in a broad range of solid tumors:
Pipeline
Designed to Shift the Paradigm
for the Treatment of Solid Tumors
We are applying our Selected TIL approach for the potential treatment of a wide range of solid tumors. We are developing a broad pipeline aimed at improving outcomes for patients with cancers, as illustrated in the chart below.
| Program | Product Overview | Key Indications | Preclinical | Phase 1 | Phase 2 | Phase 3 | Next Anticipated Milestone | |
|---|---|---|---|---|---|---|---|---|
| Selected TILs | ||||||||
| TIDAL-01 | Tumor-reactive Selected TILs |
Breast Cancer, CRC, HNSCC, Uveal melanoma |

+
|
Initial clinical data in mid-2024 | ||||
| CRC, Cutaneous and non-cutaneous melanomas, HNSCC |

+ Moffitt Collaboration*
|
|||||||
| Combination with viral immunotherapy |
Solid tumors |

|
IND submission | |||||
| TIDAL-02 | Selected TILs with next- gen manufacturing and TIL quality enhancements |
Solid tumors |

|
IND submission | ||||
| Selected TILs | |||
| Preclinical | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
TIDAL-01Tumor-reactive Selected TILs +
|
|||
TIDAL-01Tumor-reactive selected TILs 
+
|
|||
TIDAL-01Combination with viral immunotherapy |
|||
TIDAL-02Selected TILs with next-gen manufacturing and TIL quality enhancements |
|||
CRC = Colorectal cancer; HNSCC = Head and neck squamous cell carcinoma
Partners
Optimizing Technological Innovations
Through Strategic Partnerships
We are backed by successful top-tier life science and biotech investors committed to Turnstone’s growth, and have forged select collaborations with key academic institutions, major biopharmaceutical companies, elite researchers and prominent international cancer medical centers to support the development of our next-generation Selected TIL and immunotherapy pipeline.
Together with our strategic partners, we are devoted to delivering transformative therapies to the millions of cancer patients underserved by current treatment options.
Key Collaborators
In November 2022, Turnstone announced a Cooperative Research and Development Agreement with the National Cancer Institute (NCI) to study TIL therapy in novel combinations with viral immunotherapy.
News
Careers
Empowering Our People
to Drive Science Forward
Turnstone is committed to true innovation, fearless execution and operational excellence. Our team work with an unrelenting sense of urgency to fulfill our mission. Our decision-making is driven by bold science and our therapeutics are designed with the patient at the forefront of our thoughts.
We continue to build a values-driven organization that embraces diversity, equity, and recognizes that the sum of our parts drives more success than any single individual. Our talented people and deeply ingrained culture are vital elements in maintaining our competitive edge in the vast biotech universe and key to unlocking the full potential of cancer immunotherapy.
If you are passionate about making a difference for patients and are excited by our mission and science, Turnstone might just be for you.
Team Opportunities
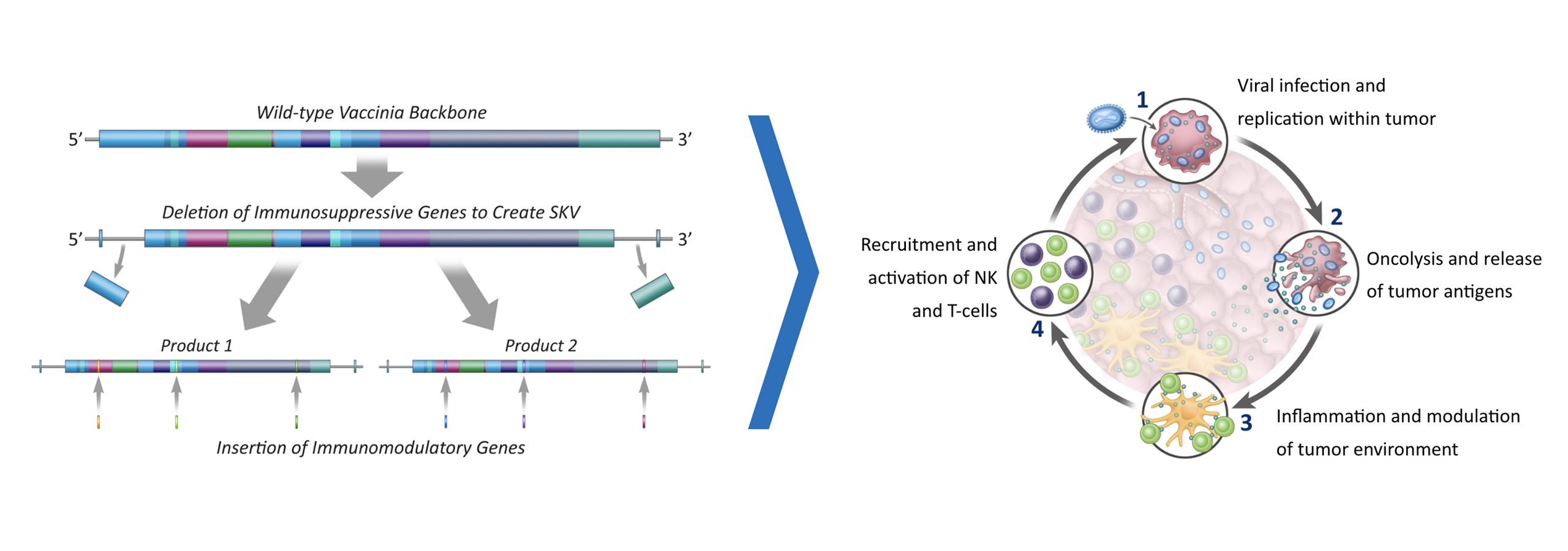
Turnstone Biologics (“Turnstone”), a public clinical stage biotech company, is developing breakthrough cancer immunotherapies by advancing two leading and complementary platforms that drive innate and adaptive tumor immunity, to provide benefit to the millions of cancer patients underserved by current treatment options. Turnstone’s proprietary vaccinia virus platform is engineered to drive coordinated immune activation, potent viral activity, and local expression of encoded therapeutics. The innovative TIL cell therapy platform leverages clinically validated treatment protocols and has been specifically designed to extend beyond the use of bulk TILs to enrich for the most relevant T-cells for tumor eradication, preserving broad antigen diversity and minimizing time to treatment for patients. The Company has an ongoing Phase 1 trial in solid tumors for the lead TIL therapy candidate, TIDAL-01, and a Phase 1 trial in partnership with the Moffitt Cancer Center.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Summary:
We are seeking a highly effective, self-motivated Scientist to join our Analytical Development team at Turnstone Biologics. In this role, you will contribute to the advancement of our cell therapy programs, particularly in developing next-generation T-cell therapies for solid tumors. The ideal candidate will be responsible for developing, qualifying, and implementing analytical methods for drug product (DP) and in-process sample characterization with minimal guidance. Key responsibilities include designing and conducting experiments, analyzing, and interpreting results, and communicating findings in team meetings. Additionally, the role requires collaboration with internal and external partners, including CDMO and CRO. The position is an onsite, lab-based role in La Jolla, CA, with responsibilities as an individual contributor, reporting to the Director of Analytical Development.
Responsibilities:
- Contribute to the development, optimization, qualification, and transfer of analytical assays, including potency and phenotypic profiling for both lot release and characterization of cell therapy products.
- Design and execute assays and characterization studies to interrogate immune cell biology and function with minimal assistance.
- Plan, coordinate, and execute analytical testing using multi-parametric flow cytometry, ELISA/Ella/MSD, and molecular methods.
- Collaborate cross-functionally and support routine Process Development (PD) sample testing and other internal/external studies supporting regulatory submission.
- Document laboratory procedures and experiments with great attention to detail, including Electronic Lab Notebook (ELN) record keeping.
- Support method transfer to partners at external contract manufacturing organizations, including compilation of documentation (protocols, test methods, reports).
- Develop, revise, approve, and own SOPs (Standard Operating Procedures) and technical (transfer, validation, bridging, etc.) protocols / reports.
- Analyze data, prepare reports and data packages for communicating with key stakeholders, senior management, and external partners.
- Willingness to travel to external partner locations for method‑related support, when necessary.
- Additional duties as assigned.
Education and Experience:
- MS with 4+ years OR PhD in Biology with 1+ years of Analytical Development experience.
- Hands-on experience with flow cytometry and ELISA-based assay development optimization, and troubleshooting is required.
- Experience with cell-based potency assessment and functional characterization of drug products is desired.
- Strong technical acumen, with a keen eye for detail in data analysis and record-keeping skills.
- Basic understanding of ICH guidelines for assay qualification/validation.
- Experience with data analysis such as GraphPad Prism, FlowJo and JMP.
- Comfortable in a fast-paced environment with minimal direction and able to adjust workload based upon changing priorities.
- Self-motivated, detail-oriented, and willing to accept temporary responsibilities outside of initial job description.
- Demonstrated ability to manage multiple tasks/projects with demanding timelines.
- Strong interpersonal, teamwork, and organizational skills; effective oral and written communication skills.
- Occasional travel to support cross-functional workshops or strategy/planning activities.
COMPENSATION
At Turnstone, we prioritize the well-being and success of our team. Join us and enjoy a competitive compensation package, including a base salary and performance-based bonuses. Our comprehensive benefits include:
- Healthcare Coverage: Medical, dental, and vision insurance for you and your dependents.
- Retirement Planning: 401(k) plan with employer contributions.
- Time Off: Generous paid time off, including vacation, sick leave, and holidays.
- Workplace Flexibility: Flexible schedules and remote work options.
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics (“Turnstone”), a public clinical stage biotech company, is developing breakthrough cancer immunotherapies by advancing two leading and complementary platforms that drive innate and adaptive tumor immunity, to provide benefit to the millions of cancer patients underserved by current treatment options. Turnstone’s proprietary vaccinia virus platform is engineered to drive coordinated immune activation, potent viral activity, and local expression of encoded therapeutics. The innovative TIL cell therapy platform leverages clinically validated treatment protocols and has been specifically designed to extend beyond the use of bulk TILs to enrich for the most relevant T-cells for tumor eradication, preserving broad antigen diversity and minimizing time to treatment for patients. The Company has an ongoing Phase 1 trial in solid tumors for the lead TIL therapy candidate, TIDAL-01, and a Phase 1 trial in partnership with the Moffitt Cancer Center.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Summary:
We are seeking a highly effective, self-motivated Senior Scientist to join our Analytical Development team at Turnstone Biologics. In this role, you will contribute to the advancement of our cell therapy programs, particularly in developing next-generation T-cell therapies for solid tumors. The ideal candidate will be responsible for innovating, designing, developing, troubleshooting, and implementing analytical methods. Key responsibilities include independently designing and conducting experiments, analyzing, and interpreting results, and effectively communicating findings in team meetings. Additionally, the role requires collaboration with internal and external partners, including CDMO and CRO. This position is suited for someone who is passionate about cell therapy and thrives in a fast-paced, collaborative environment. The successful candidate will also perform qualification/validation and tech transfer of assays, routine testing of drug product (DP) and in-process samples. The position is an onsite, lab-based role in La Jolla, CA, with responsibilities as an individual contributor, reporting to the Director of Analytical Development.
Responsibilities:
- Independently design, develop, optimize, troubleshoot, and qualify analytical methods to support drug product (DP) release and characterization ensuring technical excellence.
- Evaluate new and innovative technologies for determining drug product potency and product/process impurities.
- Plan and execute routine DP testing activities, including in-process and stability samples, using flow cytometry and ELISA/Ella to support regulatory submissions.
- Lead assay transfer to external partners and training analysts.
- Author, review and own test methods/SOPs, qualification protocols, and technical reports in accordance with regulatory guidelines.
- Document laboratory procedures and experiments with great attention to detail, including Electronic Lab Notebook (ELN) record keeping.
- Analyze data, prepare reports and data packages for communicating with key stakeholders, senior management, and external partners.
- Coordinate tasks across functions, demonstrating prioritization and planning skills.
- Present work to key stakeholders and leadership.
- Provide assistance and mentorship to junior team members.
- Willingness to travel to external partner locations for method‑related support, when necessary.
- Additional duties as assigned.
Education and Experience:
- Ph.D. in Biology with at least 4+ years of relevant industry experience.
- Strong preference for candidates with experience in immuno-oncology with a focus on engineered T cells and/or other immune cell types.
- Proficiency in a wide range of analytical techniques, including but not limited to multi-parametric flow cytometry, ELISA/MSD, and qPCR/ddPCR.
- Experience in multi-color flow cytometry analyses, including panel design and data analysis is essential.
- Experience in developing cell-based potency assay will be a plus.
- Ability to work in a highly adaptive environment and learn new skills for changing priorities.
- Knowledge of cGMP and regulatory guidelines (e.g., FDA, EMA) and a track record of ensuring compliance in analytical operations.
- Good understanding of ICH guidelines for assay qualification/validation.
- Basic understanding of method lifecycle management from development to validation and beyond.
- Ability to analyze and interpret data and contextualize with the program goals and requirements in a phase-appropriate manner.
- Demonstrated resilience, influence, relationship-building, and problem-solving skills in a variety of situations.
- Experience managing, mentoring, and engaging direct reports is a plus.
- Self-motivated, detail-oriented, and willing to accept temporary responsibilities outside of initial job description.
- Demonstrated ability to manage multiple tasks/projects with demanding timelines.
- Strong interpersonal, teamwork, and organizational skills; effective oral and written communication skills.
- Occasional travel to support cross-functional workshops or strategy/planning activities.
COMPENSATION
At Turnstone, we prioritize the well-being and success of our team. Join us and enjoy a competitive compensation package, including a base salary and performance-based bonuses. Our comprehensive benefits include:
- Healthcare Coverage: Medical, dental, and vision insurance for you and your dependents.
- Retirement Planning: 401(k) plan with employer contributions.
- Time Off: Generous paid time off, including vacation, sick leave, and holidays.
- Workplace Flexibility: Flexible schedules and remote work options.
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics is a clinical-stage biotechnology company developing new medicines to treat and cure solid tumors by pioneering a differentiated approach to TIL therapy. Turnstone’s innovative TIL therapy is based upon the identification, selection, and expansion of the most potent tumor-reactive T cells, known as Selected TILs, and is designed to overcome the limitations of first-generation bulk TILs that have demonstrated objective responses only in limited tumor types. Turnstone’s most advanced program, TIDAL-01, is currently being evaluated in two Phase 1 studies in patients with melanoma, breast cancer and colorectal cancer, and the Company is also actively advancing its preclinical pipeline programs including TIDAL-02, its next Selected TIL program, and its TIDAL-01 and viral immunotherapy combination program.
Position overview:
We are seeking an innovative, highly motivated, Analytical Leader with method development and analytical life cycle management and tech transfer experience to work on innovative cancer treatment. This leader will work with Process Development, Research, Translational, and Quality departments to provide process and analytical development and CMC leadership to a team responsible for analytical characterization of cell therapy products, maturation of research analytical methodology into development and GMP, and analytical method transfer from/ to contract vendors. They will have excellent people leadership abilities and be responsible for overseeing lab studies, analyzing experimental data, and assuring careful documentation of resultant information.
The Responsibilities include but are not limited to the following:
Experience:
- Ph.D. in Chemistry, Biochemistry, Biology, or related discipline with 10+ years of leadership experience in the biotech and/or pharmaceutical industry OR
- MS Degree. in Chemistry, Biochemistry, Biology, or related discipline with 10+ years of leadership experience in the biotech and/or pharmaceutical industry OR
- BS Degree. in Chemistry, Biochemistry, Biology, or related discipline with 12+ years of leadership experience in the biotech and/or pharmaceutical
- Well versed in various analytical techniques such as flow cytometry, ELISAs, PCR, FTIR, UV and Fluorescence spectroscopy, enzyme assays and other applicable methods to the testing of biopharmaceuticals, preferred.
- Excellent understating of cellular processes and metabolism and experience with with T-cells
- Knowledge of QbD approaches to analytical method development including DOE, risk assessment and statistical analysis
- Deep knowledge of technical writing for Post-Approval Changes, BLA, IND
- Demonstrated leadership ability in pharmaceutical manufacturing of biotechnology products, cell therapy products, analytical method development, tech transfer, and method qualification/validation and process development
- Demonstrated ability to work with both reporting organization and senior leadership teams
- Excellent interpersonal – verbal and written communication skills
- Ability to lead a diverse technical staff team with attention to detail, to think critically and demonstrate troubleshooting and problem-solving skills, and work with and lead others
- Expertise with analytical method development and qualification and analysis of cell therapy products
- Proficient in MS Word, Excel, PowerPoint, and other applications
- Comfortable in a dynamic, growing company
- Agile mindset and ability to adjust workload based upon changing priorities
- Creative and thoughtful problem-solving skills
- Able to take a proactive, solution-oriented approach
- Excellent interpersonal communication skills, including the ability to listen well and appropriately inform and guide stakeholders
- Ability to work in, lead and foster cross-functional, collaborative teams
- Ability to effectively present results and proposals to audiences of all levels
- Experience supporting, developing, and mentoring staff and creating inclusive, high-performing teams
- Someone who leads by example
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics is a clinical-stage biotechnology company developing new medicines to treat and cure solid tumors by pioneering a differentiated approach to TIL therapy. Turnstone’s innovative TIL therapy is based upon the identification, selection, and expansion of the most potent tumor-reactive T cells, known as Selected TILs, and is designed to overcome the limitations of first-generation bulk TILs that have demonstrated objective responses only in limited tumor types. Turnstone’s most advanced program, TIDAL-01, is currently being evaluated in two Phase 1 studies in patients with melanoma, breast cancer and colorectal cancer, and the Company is also actively advancing its preclinical pipeline programs including TIDAL-02, its next Selected TIL program, and its TIDAL-01 and viral immunotherapy combination program.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Job Description:
We are seeking an innovative, highly motivated, versatile leader to join us as our Clinical Regulatory Leader. This role will report to the Vice President, Clinical Development and sit will be the regulatory representative at various core teams. The successful candidate will be results-driven and a highly skilled Clinical Regulatory Leader with extensive experience in guiding regulatory strategies and ensuring compliance for pharmaceutical and biotechnology companies. The ideal candidate will be adept at navigating the complex landscape of regulatory affairs, and have a proven track record of successfully leading cross-functional teams to achieve regulatory milestones and approvals.
This is both a strategic and execution focused role requiring someone to see the big picture, plan, and roll up their sleeves and get things done. This role requires proven leadership to effectively communicate, coordinate, and collaborate cross-functionally with Clinical Operations, External Manufacturing, Quality, Technical Operations, Research, Finance, IT and Commercial teams.
Responsibilities:
-
- Provide expert, technical and professional advice, guidance and leadership to the Company on Regulatory Affairs matters.
- Proactively participate in design of global regulatory strategies for the development of cell therapy products
- Serve as one of the primary liaison between Turnstone and all strategic partners/ partner companies in regulatory topics/issues.
- Plan, oversee and manage preparation and submissions (including but not limited to pre-IND, Initial IND/CTA and amendments, Safety reporting, BLA, Orphan drug designation); this will require strong regulatory knowledge and understanding of the underlying science, cross-functional interactions, and excellent project management and writing skills
- Participate in interactions with regulatory agencies to solidify strategy and address regulatory comments
- Lead preparation of agency meetings and actively participate in scheduled meetings
- Drive adherence to regulatory requirements and guidelines
- Perform regulatory intelligence activities – monitor regulation changes and competitor trends/strategy
- Provide regulatory input regarding budget
- With Clinical Operations and Quality Assurance, ensure compliance of Clinical Development with GCPs and SOPs and other relevant regulations.
- Review and approve departmental and Clinical SOPs and revisions.
- Manage all relevant contract relationships.
- Maintain a thorough understanding of GCPs, internal SOPs, and guidance documents issued by various regulatory agencies, including the US-FDA, EMA, and ICH.
- Maintain a regulatory document system.
Education and Experience:
-
- Degree in health or life sciences, including chemistry, molecular biology, or similar. PhD preferred, Masters/Bachelors acceptable with relevant experience
- Regulatory Affairs Certification (RAC) preferred
- Background ideally oncology cell therapy or biologics
- 8 + year experience with PhD; 10+ years with Masters or bachelor’s degrees
- Proven track record of managing critical projects as a part of an interdisciplinary team
- Proven track record of representing the department in project teams, committees and external meetings
- Prior experience managing regulatory submissions to deadlines
- Thorough understanding of relevant drug development regulations and guidelines
- Outstanding interpersonal and communication (written and verbal) skills
- Effective task planning and coordination abilities
- Proficiency with computer and standard software programs (Microsoft Office, PowerPoint and Excel)
COMPENSATION
An attractive compensation package commensurate with this senior leadership role will be provided.
- To Apply:
Please click here to apply!
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics is a clinical-stage biotechnology company developing new medicines to treat and cure solid tumors by pioneering a differentiated approach to TIL therapy. Turnstone’s innovative TIL therapy is based upon the identification, selection, and expansion of the most potent tumor-reactive T cells, known as Selected TILs, and is designed to overcome the limitations of first-generation bulk TILs that have demonstrated objective responses only in limited tumor types. Turnstone’s most advanced program, TIDAL-01, is currently being evaluated in two Phase 1 studies in patients with melanoma, breast cancer and colorectal cancer, and the Company is also actively advancing its preclinical pipeline programs including TIDAL-02, its next Selected TIL program, and its TIDAL-01 and viral immunotherapy combination program.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Job Description:
We are seeking an innovative, highly motivated, versatile leader to join us as our Clinical Regulatory Leader. This role will report to the Vice President, Clinical Development and sit will be the regulatory representative at various core teams. The successful candidate will be results-driven and a highly skilled Clinical Regulatory Leader with extensive experience in guiding regulatory strategies and ensuring compliance for pharmaceutical and biotechnology companies. The ideal candidate will be adept at navigating the complex landscape of regulatory affairs, and have a proven track record of successfully leading cross-functional teams to achieve regulatory milestones and approvals.
This is both a strategic and execution focused role requiring someone to see the big picture, plan, and roll up their sleeves and get things done. This role requires proven leadership to effectively communicate, coordinate, and collaborate cross-functionally with Clinical Operations, External Manufacturing, Quality, Technical Operations, Research, Finance, IT and Commercial teams.
Responsibilities:
-
- Provide expert, technical and professional advice, guidance and leadership to the Company on Regulatory Affairs matters.
- Proactively participate in design of global regulatory strategies for the development of cell therapy products
- Serve as one of the primary liaison between Turnstone and all strategic partners/ partner companies in regulatory topics/issues.
- Plan, oversee and manage preparation and submissions (including but not limited to pre-IND, Initial IND/CTA and amendments, Safety reporting, BLA, Orphan drug designation); this will require strong regulatory knowledge and understanding of the underlying science, cross-functional interactions, and excellent project management and writing skills
- Participate in interactions with regulatory agencies to solidify strategy and address regulatory comments
- Lead preparation of agency meetings and actively participate in scheduled meetings
- Drive adherence to regulatory requirements and guidelines
- Perform regulatory intelligence activities – monitor regulation changes and competitor trends/strategy
- Provide regulatory input regarding budget
- With Clinical Operations and Quality Assurance, ensure compliance of Clinical Development with GCPs and SOPs and other relevant regulations.
- Review and approve departmental and Clinical SOPs and revisions.
- Manage all relevant contract relationships.
- Maintain a thorough understanding of GCPs, internal SOPs, and guidance documents issued by various regulatory agencies, including the US-FDA, EMA, and ICH.
- Maintain a regulatory document system.
Education and Experience:
-
- Degree in health or life sciences, including chemistry, molecular biology, or similar. PhD preferred, Masters/Bachelors acceptable with relevant experience
- Regulatory Affairs Certification (RAC) preferred
- Background ideally oncology cell therapy or biologics
- 8 + year experience with PhD; 10+ years with Masters or bachelor’s degrees
- Proven track record of managing critical projects as a part of an interdisciplinary team
- Proven track record of representing the department in project teams, committees and external meetings
- Prior experience managing regulatory submissions to deadlines
- Thorough understanding of relevant drug development regulations and guidelines
- Outstanding interpersonal and communication (written and verbal) skills
- Effective task planning and coordination abilities
- Proficiency with computer and standard software programs (Microsoft Office, PowerPoint and Excel)
COMPENSATION
An attractive compensation package commensurate with this senior leadership role will be provided.
- To Apply:
Please click here to apply!
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics (“Turnstone”), a public clinical stage biotech company, is developing breakthrough cancer immunotherapies by advancing two leading and complementary platforms that drive innate and adaptive tumor immunity, to provide benefit to the millions of cancer patients underserved by current treatment options. Turnstone’s proprietary vaccinia virus platform is engineered to drive coordinated immune activation, potent viral activity, and local expression of encoded therapeutics. The innovative TIL cell therapy platform leverages clinically validated treatment protocols and has been specifically designed to extend beyond the use of bulk TILs to enrich for the most relevant T-cells for tumor eradication, preserving broad antigen diversity and minimizing time to treatment for patients. The Company has an ongoing Phase 1 trial in solid tumors for the lead TIL therapy candidate, TIDAL-01, and a Phase 1 trial in partnership with the Moffitt Cancer Center.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Summary:
We are seeking a highly effective, self-motivated, and versatile Quality Assurance professional with demonstrated knowledge of, and experience in, Quality Systems Management. As QA Associate you will support the team with a variety of systems including but not limited to: administration of our document management system and training program as well as support for change management, deviations/CAPAs, trending and Key Performance Indicators (KPIs). You will be responsible for ensuring that such systems and changes are supported in accordance with sound change management principles and Turnstone-defined procedures. This role is critical in maintaining a strong partnership with both our internal and external stakeholders in pursuit of advancing our pipeline. This role provides support remotely across all domestic time zones and reports into the Sr Manager for Quality Operations.
Responsibilities:
- Administration of electronic systems such as Veeva and LearnGxP including supporting system updates, managing and administering training, and supporting document control activities.
- Support the trending of quality systems such as deviation, change control, and CAPA programs as well as creates, owns, and manages deviations, change control requests, and CAPA records pertaining to their functional area.
- Supports the Quality Operations function in evaluation and disposition of labelling, raw materials, intermediates and finished products through timely evaluation of batch records, laboratory results, and other GMP documents as needed.
- Provide administrative support for audit program and quality agreements.
- Reads, understands, and follows SOPs, and complies with GMP.
- Writes new standard operating procedures or revises existing documentation utilizing document management systems.
- Assists in the implementation of new processes.
- Assists in assembling quality metrics and KPIs.
- Identifies, coordinates, and implements continuous improvements.
- Prioritizes day-to-day support for their functional area and longer-term projects or investigations.
- Creates/revises QA documents (gap assessments, risk assessments, reports) in document management system.
- Facilitates and coordinates training of new and existing team members, prepares training materials as necessary.
- Represents QA, as needed, during meetings relevant to their functional area, communicates and tracks all follow-up items through to completion
- Additional duties as assigned.
Education and Experience:
- Bachelor’s in Biology, Engineering or science-related field preferred.
- 2-4+ years industry experience, preferably in gene and cell therapy.
- 2+ years of QA experience.
- Experience in GMP environments including knowledge of change management principles and industry standard QMS applications.
- Experience with Veeva, LearnGxP, DocuSign, SharePoint, SmartSheet are considered an asset.
- Demonstrated ability to manage multiple tasks/projects with demanding timelines.
- Strong interpersonal, teamwork, and organizational skills; effective oral and written communication skills.
- Occasional travel to support cross-functional workshops or strategy/planning activities.
COMPENSATION
At Turnstone, we prioritize the well-being and success of our team. Join us and enjoy a competitive compensation package, including a base salary and performance-based bonuses. Our comprehensive benefits include:
- Healthcare Coverage: Medical, dental, and vision insurance for you and your dependents.
- Retirement Planning: 401(k) plan with employer contributions.
- Time Off: Generous paid time off, including vacation, sick leave, and holidays.
- Workplace Flexibility: Flexible schedules and remote work options.
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics (“Turnstone”), a public clinical stage biotech company, is developing breakthrough cancer immunotherapies by advancing two leading and complementary platforms that drive innate and adaptive tumor immunity, to provide benefit to the millions of cancer patients underserved by current treatment options. Turnstone’s proprietary vaccinia virus platform is engineered to drive coordinated immune activation, potent viral activity, and local expression of encoded therapeutics. The innovative TIL cell therapy platform leverages clinically validated treatment protocols and has been specifically designed to extend beyond the use of bulk TILs to enrich for the most relevant T-cells for tumor eradication, preserving broad antigen diversity and minimizing time to treatment for patients. The Company has an ongoing Phase 1 trial in solid tumors for the lead TIL therapy candidate, TIDAL-01, and a Phase 1 trial in partnership with the Moffitt Cancer Center.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Summary:
We are seeking a highly effective, self-motivated, and versatile Quality Assurance professional with demonstrated knowledge of, and experience in, end to end manufacturing operations. As QA Specialist you will support the team with a variety of manufacturing processes, test methods, laboratory techniques, and material changes, improvements, and innovations. You will be responsible for ensuring that such changes and projects are executed in accordance with sound change management principles and Turnstone-defined procedures. This role is critical in maintaining a strong partnership with our contract development and manufacturing organizations (CDMOs) in pursuit of advancing our pipeline and is expected to be hands-on in the operational details of the Manufacturing and Quality support at CDMOs.
Responsibilities:
- Supports the evaluation and disposition of labelling, raw materials, intermediates and finished products through timely evaluation of batch records, laboratory results, and other GMP documents.
- Participate in the overseeing of deviation, change control, and CAPA programs as well as creates, owns, and manages deviations, change control requests, and CAPA records pertaining to their functional areas.
- Initiates and/or reviews and approves manufacturing process deviations.
- Reads, understands, and follows SOPs, and complies with GMP.
- Writes new standard operating procedures or revises existing documentation utilizing document management systems.
- Assists in the implementation of new processes.
- Electronic system usage with tools such as Veeva and LearnGxP.
- Assists supervisor by assembling metrics as requested for their functional area.
- Identifies, coordinates, and implements continuous improvements.
- Prioritizes day-to-day support for their functional area and longer-term projects or investigations.
- Integrates knowledge and experience as skilled specialist with knowledge of corporate and industry standards with respect to their functional area.
- Creates/revises QA documents (gap assessments, risk assessments, reports) in document management system.
- Facilitates and coordinates training of new and existing team members, prepares training materials as necessary.
- Represents QA, as needed, during meetings relevant to their functional area, communicates and tracks all follow-up items through to completion
- Additional duties as assigned.
Education and Experience:
- Bachelor’s in Biology, Microbiology, Engineering or science-related field preferred.
- 2-4+ years industry experience, preferably in gene and cell therapy.
- 2+ years of QA experience.
- Experience in GMP environments including knowledge of change management principles and industry standard QMS applications.
- Experience drafting and leading deviation investigations is considered an asset.
- Demonstrated ability to manage multiple tasks/projects with demanding timelines.
- Strong interpersonal, teamwork, and organizational skills; effective oral and written communication skills.
- Domestic travel up to 25% to support CDMO activities.
COMPENSATION
At Turnstone, we prioritize the well-being and success of our team. Join us and enjoy a competitive compensation package, including a base salary and performance-based bonuses. Our comprehensive benefits include:
- Healthcare Coverage: Medical, dental, and vision insurance for you and your dependents.
- Retirement Planning: 401(k) plan with employer contributions.
- Time Off: Generous paid time off, including vacation, sick leave, and holidays.
- Workplace Flexibility: Flexible schedules and remote work options.
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Turnstone Biologics is a clinical-stage biotechnology company developing new medicines to treat and cure solid tumors by pioneering a differentiated approach to TIL therapy. Turnstone’s innovative TIL therapy is based upon the identification, selection, and expansion of the most potent tumor-reactive T cells, known as Selected TILs, and is designed to overcome the limitations of first-generation bulk TILs that have demonstrated objective responses only in limited tumor types. Turnstone’s most advanced program, TIDAL-01, is currently being evaluated in two Phase 1 studies in patients with melanoma, breast cancer and colorectal cancer, and the Company is also actively advancing its preclinical pipeline programs including TIDAL-02, its next Selected TIL program, and its TIDAL-01 and viral immunotherapy combination program.
At Turnstone, the science drives decision-making and our therapeutics are designed with the patient foremost in mind. We continue to build a world-class, high-performing company that embraces being a learning organization with a sense of urgency. Imagine coming to work every day where over-communication, transparency, teaching, listening, asking, thinking, working hard, and having fun are normal – actually, expected? Imagine having a coach, mentor, peers, and partners where candid feedback, self-improvement, risk taking, failing, learning from that failing, and succeeding thereafter are what we call ‘another day at Turnstone’? If you are a passionate person, and our science excites you – this opportunity may be for you.
Job Description:
Turnstone Biologics is seeking a driven and experienced Process Engineer to support the Manufacturing Science & Technology Team (MSAT). This successful candidate will support the Turnstone manufacturing network by providing technical and scientific support of the TIDAL-01 operations process, such as: leading or supporting technology transfers, Person-In-Plant (PIP) support, root cause investigations, impact assessments, process improvements, and process monitoring. The successful candidate should be detail-oriented and enjoy working in a dynamic, high-paced environment to deliver innovative therapies to patients in need.
This is a key position requiring routine interactions, Internal and External Manufacturing Operations, MSAT, Process Development, Quality Assurance, Quality Control, and Supply Chain. The role will support the Turnstone Manufacturing team, based in both Ottawa, CAN and San Diego, California, and external manufacturing partners. As such, flexibility to provide remote support for manufacturing operations is required. In addition, this candidate will support the evaluation of new or future external and internal manufacturing capabilities.
Responsibilities:
• Interact with Manufacturing leadership to influence strategic and technical guidance on ongoing clinical production
• Provide on-site/remote Subject Matter Expert (SME) support for GMP operations at CMOs as Person-In-Plant (PIP)
• Supporting investigations from process deviations and impact assessments, identifying appropriate subsequent CAPAs for clinical MFG productions
• Communicate with upstream Clinical Operations and downstream Clinical Manufacturing teams, ensuring clear flow of communication across the manufacturing lifecycle
• Collaborate with Process Development teams on implementation of process optimizations, and new technology, critical reagents, and materials
• Write and review technical documentation (batch records, SOPs, protocols & reports)
• Author impact assessments in support of deviations and change controls
• Support process related deviations and provide technical support to manufacturing
• Support process improvement activities involving cross-functional teams including Manufacturing, Quality, and Process Development
• Perform risk assessments and investigations including root cause analysis utilizing a systematic approach and industry best practices
• Determine corrective and preventative actions for process-related deviations
• Support technology transfers and execution of verification runs
• Support process FMEA
• Participate and report to a cross-functional development team to advance production activities
• Ensure successful manufacturing production runs by assessing risk, setting preventative measures in place, investigating, and troubleshooting equipment and process issues
Education and Experience:
-
- • Bachelor’s degree, in life sciences, engineering or related field and 4+ years of industry experience; Masters degree with 3+ years of experience; PhD and 1+ years of experience
- • 3+ years experience with the manufacturing and operational complexity of cell therapy-based therapeutics such as TIL, CAR-T, TCR, or stem cell products is required.
- • Enjoy working in a fast-paced environment, able to manage competing priorities effectively and adapt to changing priorities
- • Prior experience with GMP manufacturing to enable support of person-in-plant and manufacturing workflows
- • Experience managing external relationships such as CMO partners or clients as a CMO provider, with a mature and thoughtful communication approach to support strong partnerships
- • Be able to travel to perform and complete listed responsibilities.
- • Experience in Process Development, Manufacturing, and/or Manufacturing Science and Technology (MSAT) for cell or gene therapies preferred
- • Strong communication skills, both written and oral
- • Strong interpersonal skills and a communication style that matches the Turnstone style: respectful, transparent, and constant
- • This role will be formally positioned out of Memphis, TN or San Diego, CA although consideration will be made for remote candidates in North America
The anticipated salary range for this role is $120,000 – $145,000.
To Apply:
Turnstone Biologics welcomes and encourages applications from people with disabilities. Accommodations are available on request for candidates taking part in all aspects of the selection process. We thank all applicants for their interest, however, only those selected will be invited for an interview.
Contact
California
9310 Athena Circle
Suite 300
La Jolla, CA 92037
USA
(347) 897-5988
Ontario
12 York Street
3rd Floor
Ottawa, ON K1N 5S6
CANADA
(613) 421-8930